Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sano, Yuichi; Sakamoto, Atsushi; Takeuchi, Masayuki; Suzuki, Hideya*; Matsumura, Tatsuro; Kawanobe, Kazunori*; Asano, Shusaku*; Maki, Taisuke*; Mae, Kazuhiro*
no journal, ,
The mass transfer coefficients during the extraction and back-extraction of lanthanide elements in the solvent extraction process using new extractants (TDdDGA, HONTA) developed for minor actinides (MA) recovery were evaluated. In the TDdDGA system, it was confirmed that the mass transfer coefficients during back extraction were improved by the addition of alcohol to the solvent, etc., and in the HONTA system, the mass transfer coefficients were relatively small.
Sasaki, Yuji; Yoshizuka, Kazuharu*
no journal, ,
It is important to study the solvent extraction of Cs, therefore the study on the diluent for the crown compounds is necessary. In this work, we investigate the physical properties of diluents and Cs extraction using the extraction solvents. The results indicate that distribution of Cs is higher when diluent having higher dielectric constant is used. Furthermore, the results using 2-nonanone, which have high dielectric constant, give the strong dependence of D(Cs) on nitric acid concentration.
Sasaki, Yuji; Matsumiya, Masahiko*
no journal, ,
DGA (diglycolamide) compounds can extract lanthanide (Ln) elements in nitric acid and shows the high D(Ln). The separation factors (SF) between light and heavy Ln are relatively high, thus the mutual separation of Ln can be easily done by DGA compounds. On the other hand, their SF is not so high, the separation and recovery between Nd and Dy are performed using batchwise multi-step extraction. In this work, calculation results are compared with the experiments, and its effect is confirmed.
Morita, Keisuke; Suzuki, Hideya*; Matsumura, Tatsuro
no journal, ,
no abstracts in English
Tsuchida, Yusuke; Matsumiya, Masahiko*; Sasaki, Yuji; Akama, Takeshi*; Arita, Yuji*
no journal, ,
We study the solvent extraction of soft acid metals, e.g., platinum metals, gold and silver from nitric and hydrochloric acids by commercially available organic compounds with low solubility in water. We would like to know some of the physical parameters obtained from DFT calculations can support the experimental results. The present work show that the extractants having S-donor give high extractability for gold.
Suzuki, Hideya*; Ban, Yasutoshi; Tsubata, Yasuhiro; Tsutsui, Nao; Toigawa, Tomohiro; Kurosawa, Tatsuya*; Shibata, Mitsunobu*; Kawasaki, Tomohiro*; Matsumura, Tatsuro
no journal, ,
The Japan Atomic Energy Agency (JAEA) has been studying partitioning technology. Recently, JAEA proposed a new liquid-liquid extraction technology called SELECT process to separate minor actinide (MA) from high-level liquid waste for transmutation. In this process, new extractants (HONTA, ADAAM) with highly practical and high extraction ability for MA was developed. A mixed solvent of HONTA and ADAAM was tested for mutual separation of MA and rare earth elements (RE). In this test, separation of MA and RE was achieved with very high yield. Furthermore, Americium (Am) and Curium (Cm) were separated efficiently with high separation factor values.
Sakamoto, Atsushi; Sano, Yuichi; Takeuchi, Masayuki
no journal, ,
no abstracts in English
Motokawa, Ryuhei
no journal, ,
no abstracts in English
Shimojo, Kojiro
no journal, ,
The applicant will give the award lecture at the 38th Annual Meeting of The Japan Association of Solvent Extraction to win The Award for Outstanding Young Researcher of 2019. The award title is "Studies on innovative extraction separation systems based on specific properties of novel extractants and ionic liquids". The applicant will talk about a series of research results on the development of novel extractants and solvent extraction methods using ionic liquids.
Okamura, Hiroyuki; Ueda, Yuki; Motokawa, Ryuhei; Mu, J.*; Masters, A. J.*; Antonio, M. R.*
no journal, ,
Under practical liquid-liquid extraction conditions with high solute concentrations, clusters of metal-extractant complexes can form in the organic phase. In these conditions, application of the slope analysis method for determining metal-extractant stoichiometry becomes difficult, and the deviations from ideal appear as non-linear responses and non-integer slopes. In this study, we developed novel extraction equilibrium analysis for practical liquid-liquid systems with consideration of the cluster formation and investigated the extension of the classical equilibrium analysis. Molecular dynamics (MD) simulation snapshots indicate the formation of aggregated clusters of 1 to 9 Zr(NO )
) (TBP)
(TBP) complexes in
complexes in  -octane, leading to the determination of composition and molar fraction of each cluster. Considering the extraction equilibrium of clusters composed of
-octane, leading to the determination of composition and molar fraction of each cluster. Considering the extraction equilibrium of clusters composed of 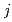 Zr(NO
Zr(NO )
) (TBP)
(TBP) complexes, the extraction equilibrium constants K
complexes, the extraction equilibrium constants K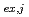 (
(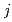 = 1-9) were calculated from the distribution ratio and the molar fraction for each cluster obtained by the MD analysis. The distribution curve calculated from the obtained K
= 1-9) were calculated from the distribution ratio and the molar fraction for each cluster obtained by the MD analysis. The distribution curve calculated from the obtained K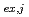 values agrees well with the experimental values. Therefore, MD simulation can accurately reproduce the experimental values in the clustering/aggregation liquid-liquid extraction system, which enabled us to extend the classical equilibrium analysis.
values agrees well with the experimental values. Therefore, MD simulation can accurately reproduce the experimental values in the clustering/aggregation liquid-liquid extraction system, which enabled us to extend the classical equilibrium analysis.
 solution; Comparison with tri-
solution; Comparison with tri- -butyl phosphate
-butyl phosphateUeda, Yuki; Kikuchi, Kei; Sugita, Tsuyoshi; Tokunaga, Kohei; Motokawa, Ryuhei; Naganawa, Hirochika
no journal, ,
The extraction and separation of Zr(IV) as a major fission product from aqueous HNO solutions have been investigated extensively because the presence of Zr in the TBP-based PUREX process for reprocessing spent nuclear fuel is problematic. Moreover, the formation of liquid-liquid (water, oil) interfacial cruds or a third phase during Zr extraction by TBP from HNO
solutions have been investigated extensively because the presence of Zr in the TBP-based PUREX process for reprocessing spent nuclear fuel is problematic. Moreover, the formation of liquid-liquid (water, oil) interfacial cruds or a third phase during Zr extraction by TBP from HNO aqueous solution occurs with a high metal loading in the organic phase. In this study, we develop a fluoroalkylated phosphate (TFP) for Zr(IV) extraction to increase the extractability and prevent third-phase formation. We investigated the extraction performance and mechanism of TFP extraction of Zr(IV) from HNO
aqueous solution occurs with a high metal loading in the organic phase. In this study, we develop a fluoroalkylated phosphate (TFP) for Zr(IV) extraction to increase the extractability and prevent third-phase formation. We investigated the extraction performance and mechanism of TFP extraction of Zr(IV) from HNO solutions into perfluorohexane and compared them with the conventional organic extraction system using TBP in n-octane. The Zr(IV) extraction strength of TFP in the fluorinated diluent perfluorohexane was much higher than that of TBP in n-octane.
solutions into perfluorohexane and compared them with the conventional organic extraction system using TBP in n-octane. The Zr(IV) extraction strength of TFP in the fluorinated diluent perfluorohexane was much higher than that of TBP in n-octane.
Kikuchi, Kei; Ueda, Yuki; Sugita, Tsuyoshi; Naganawa, Hirochika
no journal, ,
Fluorous solvents, such as perfluorinated alkanes, ethers, and tertiary amines have excellent chemical properties, including low toxicity, low viscosity, nonflammability, chemical stability, and immiscibility with both water and organic solutions. However, there are few reports on metal ion extraction in fluorous biphasic systems, because hardly any extractants that have a high affinity for fluorous solvents and the introduction of fluorous groups can reduce the extraction performance. In this study, a fluorous extractant with a sufficiently long spacer between the coordinating group and fluoroalkyl chain can maintain the original coordinating ability, which is comparable with a conventional organic extractant, and have an affinity for the fluorous solvent. We investigated the extraction performance and mechanism of TFP extraction of Zr(IV) from HNO solutions into perfluorohexane and compared them with the conventional organic extraction system using TBP in n-octane.
solutions into perfluorohexane and compared them with the conventional organic extraction system using TBP in n-octane.